
For me, 2024 is a year of introspection and growth.
I’m redefining my professional role and optimizing my own health. The two are intertwined under the umbrella of “me-search,” which involves using myself as a subject in a series of N=1 trials to better understand the latest healthcare techniques.
Being on the “cutting edge” of medicine requires a critical approach to evaluating and selecting medical options. Unfortunately, the metaphor of a sharp instrument doesn’t accurately describe the current landscape. It’s the opposite — an amorphous blob better represents the rapidly growing pool of heavily hyped, minimally evaluated medical treatments. Strolling down the supplement aisle or googling “weight loss” unleashes an avalanche of options.
Separating the wheat from the chaff isn’t easy.
For example, my new primary care doctor recommended I take a fish oil supplement to protect my heart. I trust her advice, but I also wanted to review the current medical evidence for fish oil supplements. Like many dietary supplements, the general consensus has swung back and forth.
Our story begins on a frigid ice floe in the North Atlantic…
Early Fish Oil Studies
In the 1970s, researchers noted that the native population of Greenland had lower rates of cardiac disease than those in Denmark or the United States, despite a diet much higher in saturated fats. These epidemiological findings led to the idea that people in Greenland who ate more marine vertebrates (whale, seal, and fish) had higher levels of n-3 fatty acids, which made them less likely to get heart disease.
In 1985, a new study demonstrated the cardioprotective effects of n-3 fatty acids in fish. A randomized cohort of heart attack survivors either ate two meals of oily fish a week or not. After two years, the group that ate oily fish had a 29% lower all-cause mortality rate than the group that didn’t.
Researchers then attempted to assess the benefit of n-3 fatty acids as a dietary supplement rather than through fish consumption.
Before exploring more recent fish oil studies, I want to describe some methodological challenges in conducting high-quality nutritional research.
The Challenges of Medical Research
The preponderance of poor-quality research has made nutritional advice the laughingstock of Western medicine. The frequent back-and-forth of conflicting advice makes the average politician appear steadfast:
Eggs are good for you. Eggs are bad for you. Don’t eat butter; eat margarine. Don’t eat margarine — it’s loaded with trans-saturated fat that will kill you. Calcium supplements make your bones strong. Calcium supplements are bad for your heart.
Flawed research methodologies generate conflicting advice.
Most nutritional research relies on epidemiological studies, observing who consumes what and the outcomes. This type of research has advantages: You can study many people, and more people will volunteer to fill out a questionnaire every year than eat prepackaged food for every meal for six weeks.
Epidemiological studies are also easier and less expensive, allowing them to run for many years. The study subjects represent the general population instead of the few people willing to volunteer for an interventional study.
However, dietary epidemiological studies have significant shortcomings. Can you remember everything you ate yesterday? Six weeks ago? Six months ago? Self-reported dietary questionnaires are a notorious source of error.
Also, individuals who may have suffered a bad outcome, such as cancer, can remember in much greater detail what they may have eaten in the past, introducing another form of bias.
However, the largest source of error stems from the principle that association does not equal causation. A study might find that ice cream consumption is associated with a higher risk of drowning, but this doesn’t mean ice cream consumption causes drowning. Rather, both are more likely to occur during hot weather.
There can be confounders: variables associated with both the possible risk factor and the clinical outcome being studied.
Epidemiological literature is replete with examples of supplements such as vitamin E and folate that purportedly reduce the risk of heart disease and antioxidant supplements that reduce the risk of cancer. They frequently overlook the potential “healthy lifestyle” confounder.
An individual who chooses to take a vitamin E or antioxidant supplement is often very different from someone who doesn’t take either supplement. The decision to take any supplement can be a marker for a whole host of other choices associated with better health outcomes — more sleep, less alcohol intake, more exercise, and an overall interest in maximizing one’s health.
Thus, the supplement didn’t cause a better health outcome per se. Rather, taking the supplement was a marker for variables associated with a better health outcome.
To create better nutritional studies, researchers have turned to the gold standard for interventional trials: the placebo-controlled, double-blind, randomized controlled trial.
What Is a Randomized Controlled Trial?
In a randomized controlled trial, half the participants are randomly assigned to receive the intervention and the other half to receive a placebo. Neither the study subject nor the researchers are aware of the treatment allocations.
The confounder problem is eliminated because, with a large enough study population, the randomization process should create two equivalent groups. Any confounders would be evenly distributed between the two groups and not contribute to a net difference in effect. This methodology forms the foundation of our assessment of drug therapies.
However, there is one tremendous difference between a randomized controlled drug trial and a nutritional supplement: Before starting a drug trial for a cholesterol-lowering medication such as rosuvastatin, none of the study participants would have rosuvastatin in their bodies.
That is not the case in many nutritional intervention studies, where study subjects have a range of baseline values. (Please see the appendix below for a more detailed discussion.)
For example, a study of vitamin D supplementation, a hormone the body makes in response to sunshine, may have a very different result when conducted in Norway, near the Arctic Circle, than in Costa Rica, near the equator. One could argue that the variability in baseline values shouldn’t be a factor because they’re the same in the intervention and control groups. However, this phenomenon can still critically weaken the studies and, I believe, heavily contribute to the uncertainty in interpreting fish oil supplementation studies.
In statistics, “power” refers to the likelihood of detecting a difference between study groups, should one exist. In practical terms, when designing a study, the smaller the effect size you hope to detect, the more study subjects you need to demonstrate a statistically significant result. The opposite is true for larger effect sizes.
Intuitively, this makes sense. If a hypothetical antibiotic cured 90% of everyone who received it compared to only 20% of those receiving a placebo, not as many study subjects would be needed.
However, a blood pressure medication that only reduced systolic blood pressure by a few points would require many study subjects to demonstrate a difference with statistical significance.
Whenever a study concludes that there was no treatment effect, was there actually no treatment effect, or was the study “underpowered” (that is, there weren’t enough study subjects for the magnitude of clinical benefit to demonstrate statistical significance)?
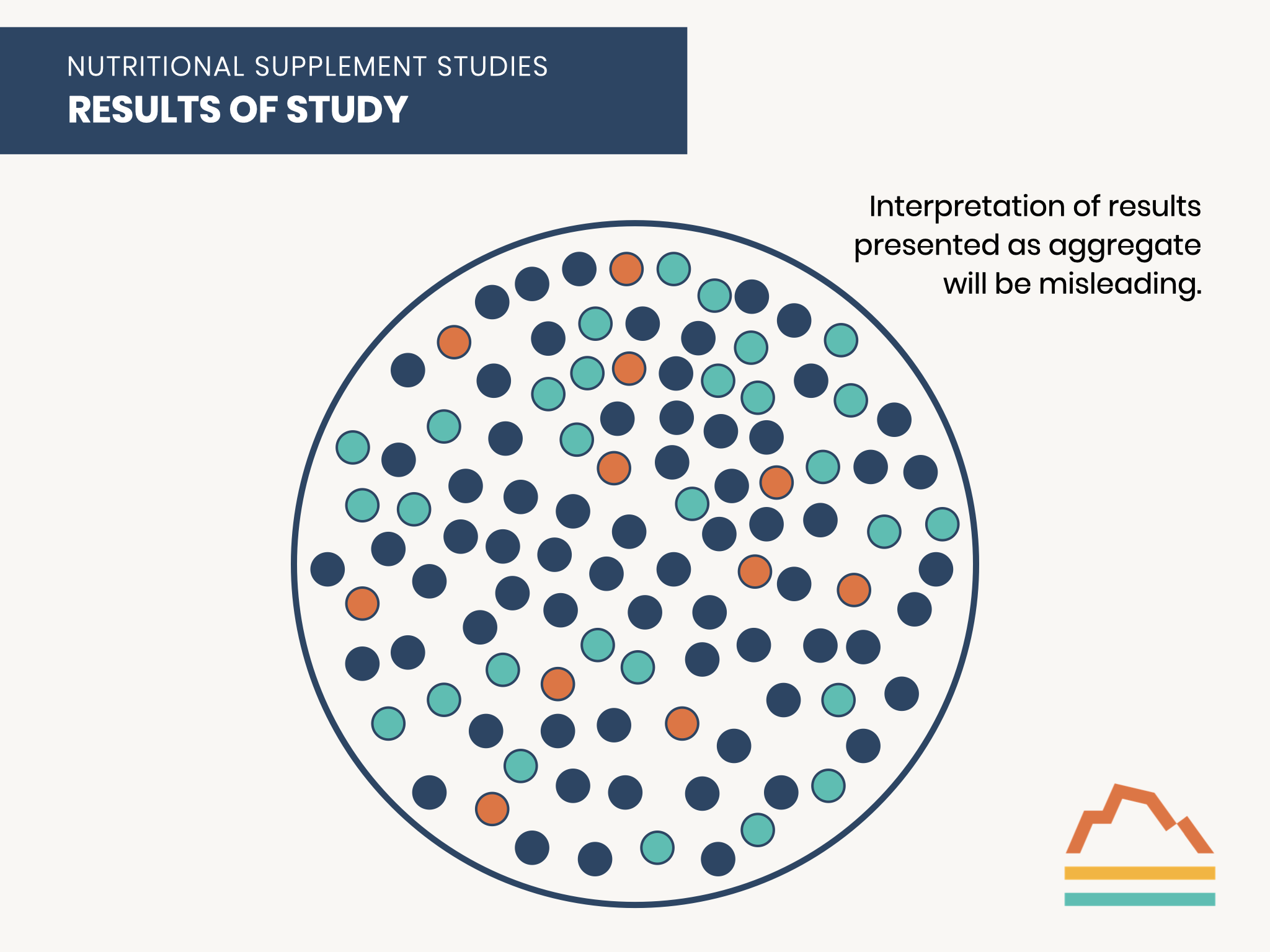
Let’s return to a hypothetical cohort of individuals enrolled in a nutritional supplement study, such as for fish oil. The study subjects are divided into three subsets based on their starting baseline level of n-3 fatty acids, the active ingredient in fish oil:
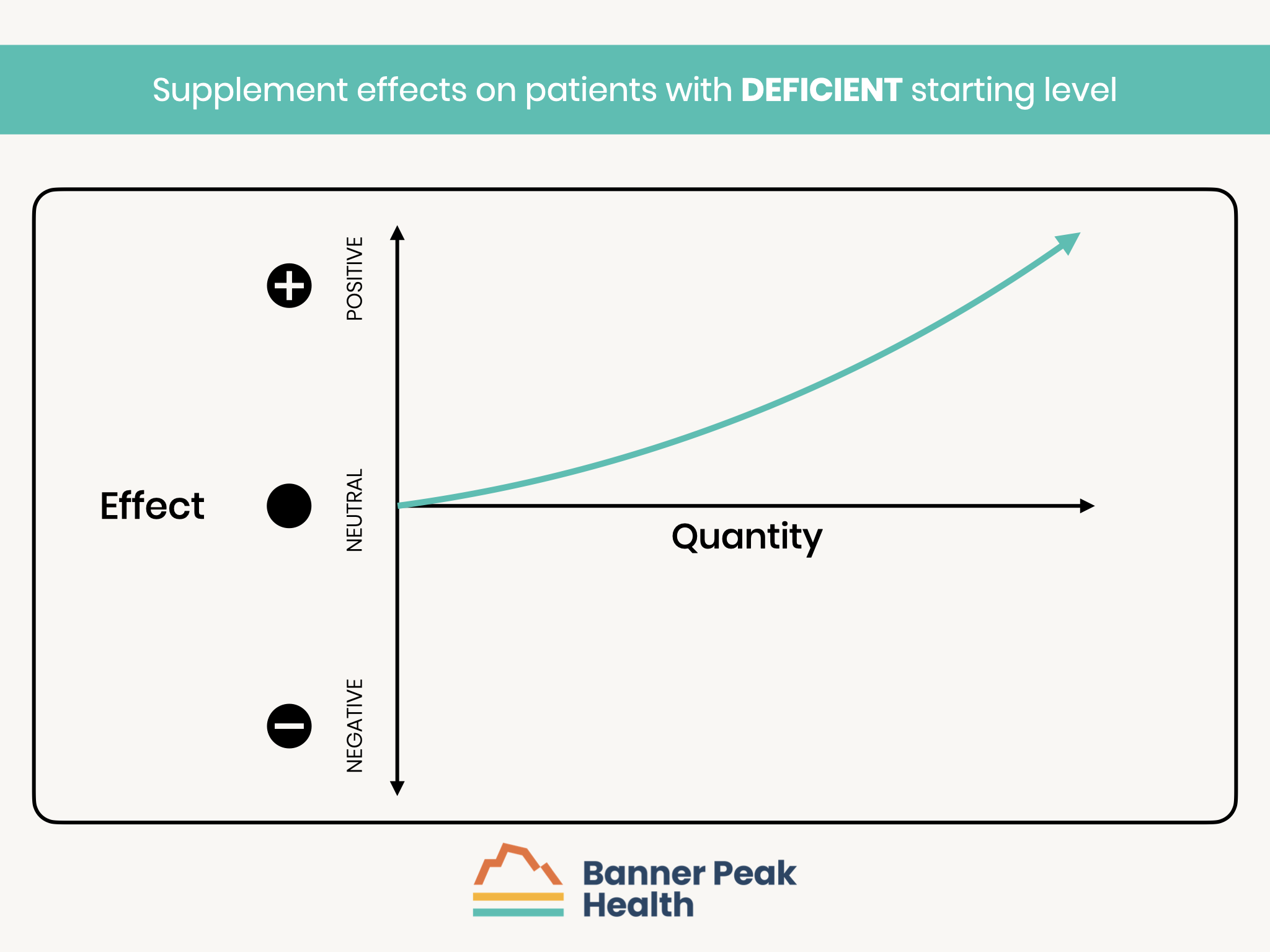
- Those with a deficiency of n-3 fatty acids are expected to have the greatest benefit from supplementation.
- Those with a normal level of n-3 fatty acids may receive little or no benefit from supplementation.
- Those with an excess of n-3 fatty acids may suffer side effects from further supplementation.
Ideally, you would measure each participant’s baseline level, categorize them into a subset, and study each group’s physiological responses separately.
Fish oil studies haven’t followed such protocols, but some offer interesting clues.
The VITAL Study
The VITAL study, published in the prestigious New England Journal of Medicine in 2019, became the definitive study for evaluating fish oil supplementation’s effect on cardiovascular disease and mortality.
With careful methodology and over 25,000 study participants, the study concluded that “omega-3 fatty acid supplementation did not reduce major cardiovascular events,” which became the prevailing wisdom.
Based on that study, I stopped taking fish oil supplements. However, that much-publicized conclusion masked a very different story.
First, the study demonstrated no significant difference in treatment groups for major cardiovascular events (the cumulative total of myocardial infarction, stroke, or death from cardiovascular causes). Yet, some outcomes showed benefits of fish oil supplementation: a 28% reduction in myocardial infarction (MI) and a 50% reduction in death from an MI.
The analysis I was most interested in was hidden in the supplemental material (available separately online but not included in the published document): The study participants weren’t stratified according to their n-3 fatty acid baseline levels.
However, they were analyzed according to whether they consumed more or less than an average of 1.5 fish meals per week, which would be expected to correlate with baseline levels. Out of 25,435 study participants, 13,514 reported eating less than an average of 1.5 fish meals per week.
The less frequent fish eaters showed a statistically significant 19% reduction in major cardiovascular events — the opposite conclusion of analyzing the entire cohort. By analyzing the subgroup of the population with a probable deficiency, the study demonstrated a statistically significant improvement with supplementation. This proves my point that the key to conducting a meaningful supplement study is to analyze those who actually have a deficiency.
When assessing whether to implement findings from a research study, “generalizability” becomes important — that is, are the participants and treatments evaluated in a particular study comparable to the patient sitting in front of you in the clinic? For example, a treatment that works great for young military recruits may not be the best choice for a frail 90-pound woman.
The VITAL study results are generalizable: if you have a suspected or documented low level of n-3 fatty acid, fish oil supplements are proven to reduce your risk of major cardiac events. Unfortunately, the press popularized the opposite conclusion.
Since the VITAL study’s publication, several large meta-analyses of fish oil supplementation for cardiac health have been published that support fish oil’s benefits. (A meta-analysis combines the data from multiple studies to create a much larger cohort to analyze.) This increases the study’s power and the ability to find statistically significant treatment effects.
Again, this supports my contention that early fish oil studies were effectively “too small” because only a subset of the participants were deficient in n-3 fatty acids and could be expected to benefit from supplementation.
My primary care doctor felt my n-3 fatty acid levels were borderline. In the context of my elevated blood pressure and borderline cholesterol levels, she believes fish oil supplementation will safely and effectively reduce my risk for adverse cardiac outcomes (in addition to other prescribed medications).
Fish oil supplements are very safe. Side effects are usually limited to gastrointestinal events such as indigestion and “fishy-tasting” belches. Fish oil has a very mild anticoagulant effect, which is one of the mechanisms for its beneficial effects. However, if you’re already on a prescribed blood thinner such as Plavix, Eliquis, or Xarelto, please ask your doctor about the risks and benefits of adding fish oil.
Some fish oil preparations contain mercury, a toxic metal. Unfortunately, our oceans are so polluted with mercury that it’s become part of the marine food chain.
Even the smallest creatures, such as plankton, can accumulate mercury. The fish that eat the plankton concentrate the mercury, and the bigger fish that eat the smaller fish continue to concentrate the mercury. Thus, larger fish, such as tuna and swordfish, have higher mercury levels than smaller fish, such as anchovies and sardines.
Higher-quality fish oil manufacturers use exclusively smaller fish and don’t have problems with mercury contamination.
I’ve begun taking fish oil from Nordic Naturals. They use only small fish and manufacture high-quality products, and their capsules don’t need to be refrigerated until after opening the bottle.
Fish oil can degrade if stored too long in a warehouse or exposed to extreme heat during shipping. I recommend ordering directly from Nordic Naturals — they’re careful about how they store and ship their products.
At Banner Peak Health, we strive to be at the forefront of healthcare, particularly prevention. We’re always looking for aspects of cardiovascular risk to identify and mitigate. Low levels of n-3 fatty acids are an important risk factor that can be safely treated with fish oil supplementation.
Appendix:
Many randomized controlled trials of dietary supplements suffer from the design flaw of aggregating all study participants regardless of their baseline levels.
This population can more accurately be considered as composed of three sub-groups: those with deficient, normal, or excess levels before any supplementation.
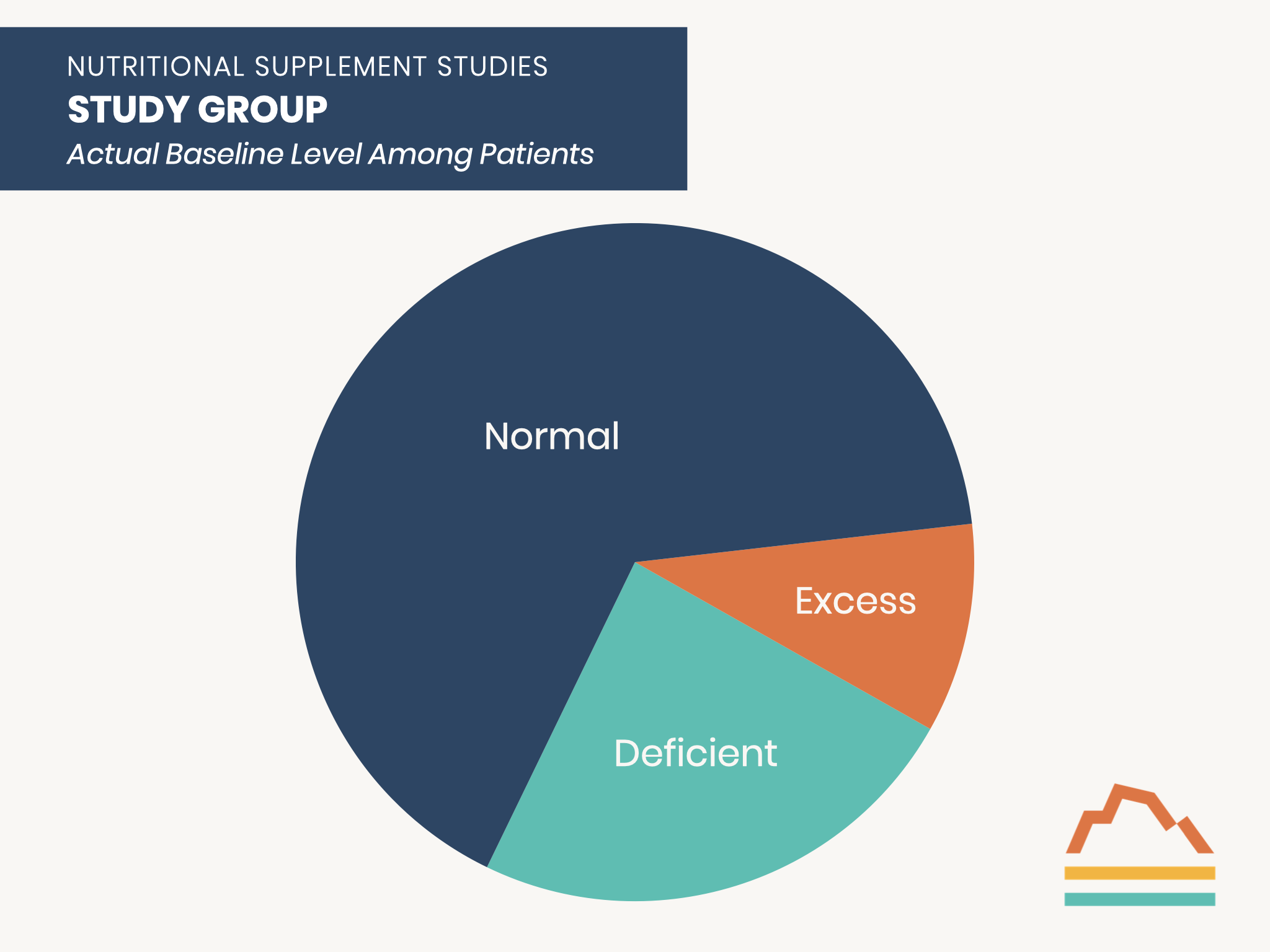
Those who are deficient at baseline are the ones who can benefit from supplementation.
Those with normal baseline levels would NOT be expected to benefit from supplementation.
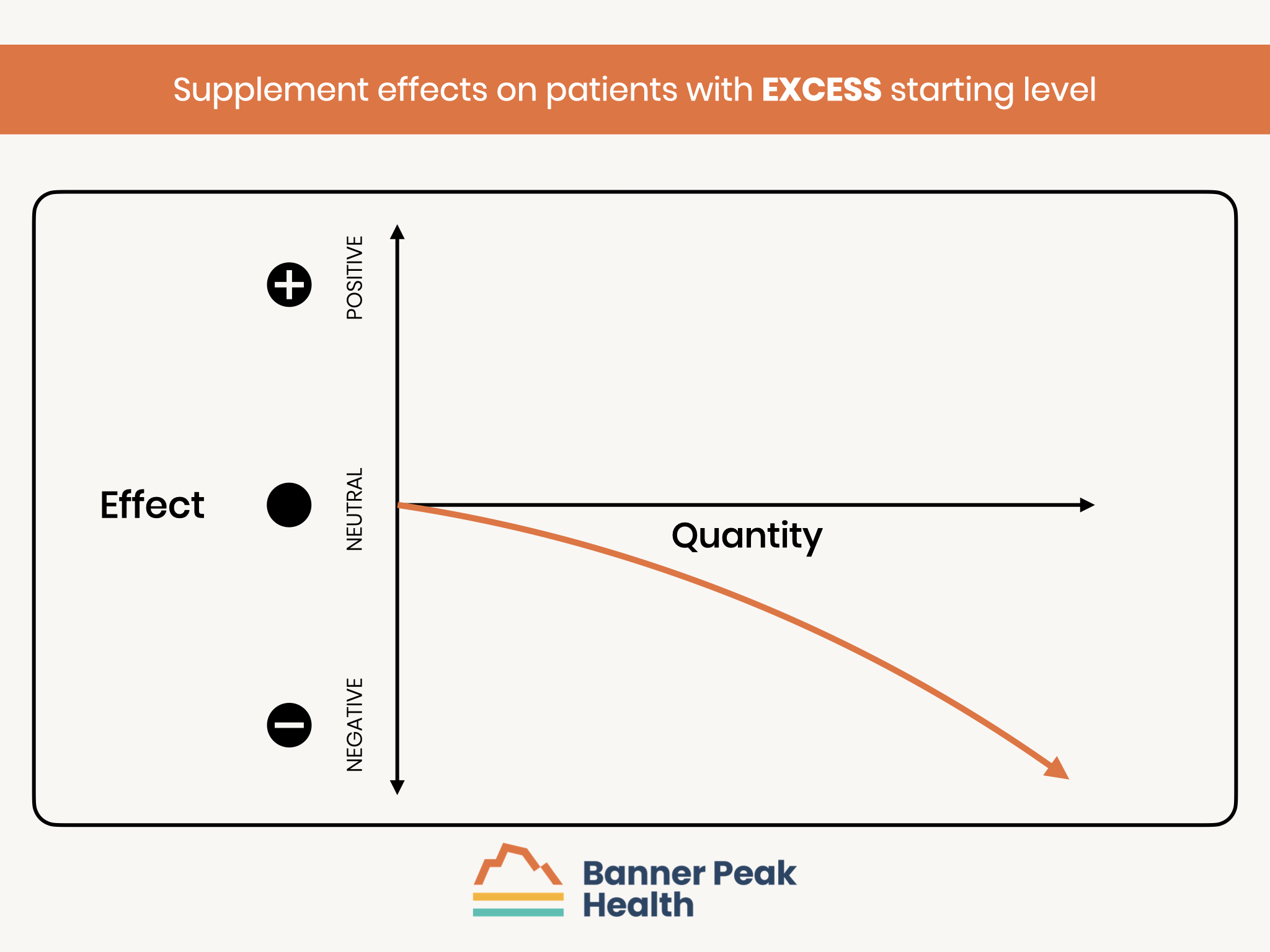
Those with excess levels at baseline are at risk of a worse outcome with additional supplementation.
Therefore, an analysis of the entire cohort may not have the statistical power to detect a treatment benefit because the number of participants with the possibility of improvement may be too small to reach statistical significance.

Barry Rotman, MD
For over 30 years in medicine, Dr. Rotman has dedicated himself to excellence. With patients’ health as his top priority, he opened his own concierge medical practice in 2007 to practice medicine in a way that lets him truly serve their best interests.



